Based across the UK, including Northern Ireland, our highly skilled technicians provide hands-on support to ensure your operations run smoothly, efficiently, and compliantly.
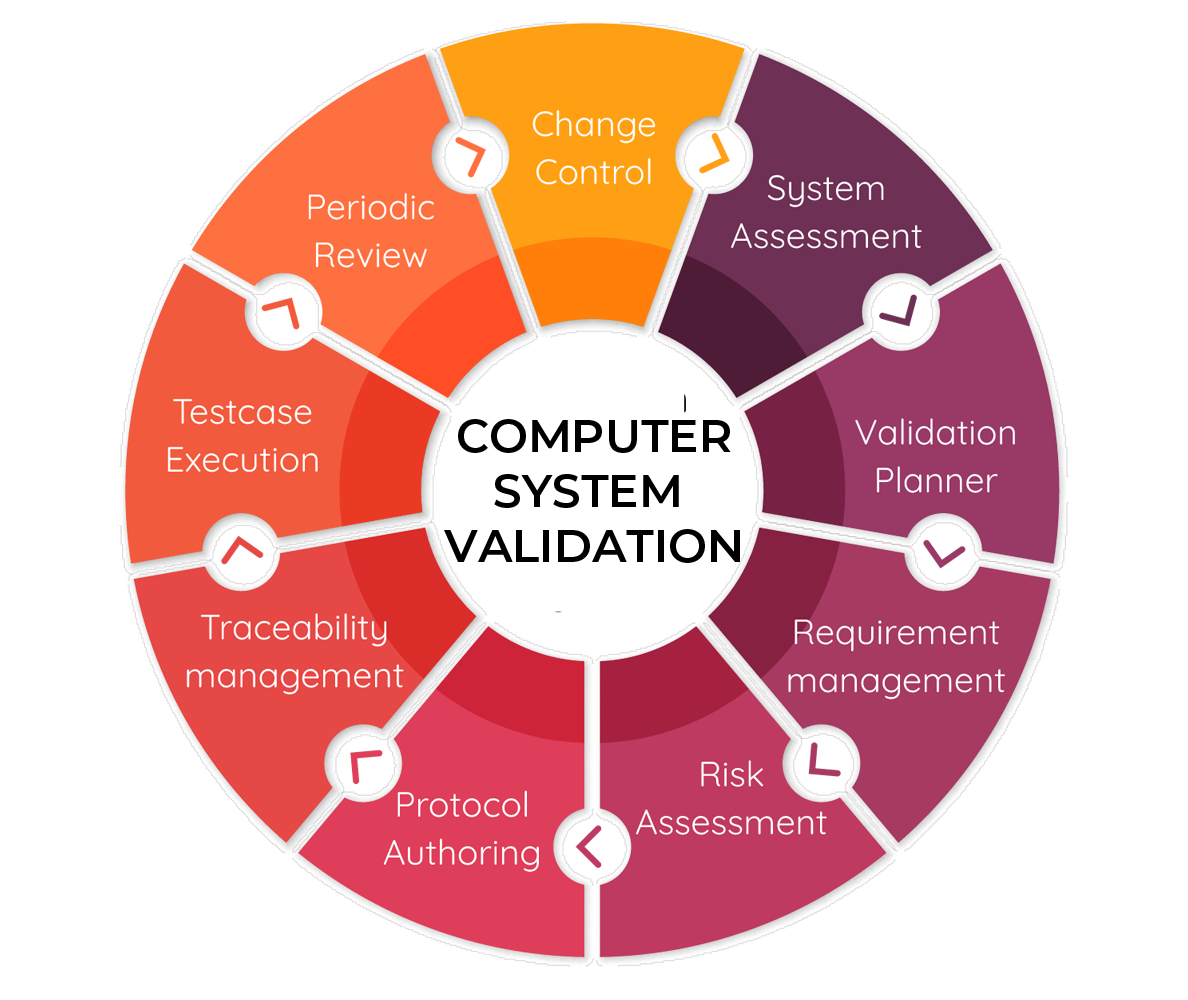
WHAT IS CSV VALIDATION?
Computer Systems Validation (CSV) - have paved the way for organisations to implement the international legal requirements for product quality & data integrity. Which in turn plays a vital role to patient safety in the pharmaceutical and medical sector.
Our CSV as a service will ensure to tick all the boxes required by EMA & FDA for your organisation to be compliant with the guidelines set out by industry regulators.
Our accredited experts are able to take on the full burden of validating your organisations computer systems, or can work collaboratively with your IT department.
Validation Planning:
Tailored strategies to meet your unique needs.
System Testing
Rigorous testing protocols to ensure reliability.
Documentation:
End-to-end documentation, including protocols and reports.
Regulatory Compliance:
Expertise in EU and international regulations (e.g., GAMP 5, FDA 21 CFR Part 11).

Our Areas of Expertise
| Industry | Why CSV validation is Needed | Example Applications |
|---|---|---|
| Pharmaceuticals & Biotechnology | Regulatory compliance (e.g., FDA 21 CFR Part 11), data accuracy for drug development. | LIMS, MES, QMS |
| Medical Devices | Adherence to ISO 13485, FDA regulations, EU MDR, ensures reliable device software and testing systems. | Product lifecycle management systems, calibration software, complaint handling systems. |
| Food & Beverage | Compliance with safety standards (ISO 22000, FDA), maintains traceability and quality. | ERP, recipe and formulation software, traceability systems. |
| Manufacturing & Automation | Ensures reliability of automated processes, protects software-related production. | SCADA systems, DCS, production planning software. |
| Clinical Research & CROs | Guarantees data accuracy and integrity for clinical trials under GCP guidelines. | EDC, CTMS, statistical analysis software. |
| Healthcare & Hospitals | Protects patient data, ensures compliance with HIPAA, GDPR. | EMR, RIS, LIS. |
| IT & Data Centres | Validates data systems for integrity, security, and compliance. | Backup/recovery systems, virtual infrastructure management, cloud-hosted enterprise systems. |
| Chemical & Petrochemical | Ensures precision and safety in data management for chemical processing. | Environmental monitoring systems, batch process control, laboratory testing software. |
| Financial Services & Banking | Validates systems handling large transactions, ensures regulatory compliance. | Fraud detection systems, risk management software, enterprise IT management tools. |
| Telecommunications & Technology | Ensures reliability and performance in critical communication infrastructure. | Network management software, subscriber billing systems, CRM tools. |

Why Choose QCOM Ltd?
-
- Experienced Team: Our technicians bring years of on-the-floor expertise in Ireland’s tech landscape.
-
- Tailored Solutions: We adapt to your specific operational requirements.
-
- Regulatory Excellence: Ensuring you remain compliant with the latest standards.
-
- Local Presence: Fast, responsive support from technicians based right here in Ireland.
As Qcom, we go beyond being a mere service provider – we become your trusted partner in achieving your IT goals.
At Qcom, Regulatory Compliance means ensuring your systems comply with international and EU regulations, such as GAMP 5, FDA 21 CFR Part 11, and others. We bring deep expertise to help you navigate complex regulations, ensuring your systems are both secure and compliant.
Qcom stands out because we offer tailored validation services that address the specific needs of your business. With in-depth knowledge of EU and international regulatory standards, we provide practical solutions to ensure your systems remain compliant and secure.
At Qcom, we support compliance by ensuring that your system meets the latest regulatory standards. We help implement the necessary processes to stay compliant with industry regulations such as GAMP 5 and FDA 21 CFR Part 11, offering peace of mind and operational security.
At Qcom, we hold Specialised knowledge of Irish and EU compliance requirements, including GAMP 5 and GDPR mandates.
DEDICATED TEAMS:
INFRASTRUCTURE:
SOFTWARE:
CLOUD:
DATA :
CONTINUITY: